SIMulation of tools / tissues interactions for the PLanning of Endovascular devices implantation.
Patient specific and predictive numerical simulation for decision support in the planning of cardiovascular interventions
Coordinator: Pascal Haigron – LTSI Laboratoire Traitement du Signal et de l’Image
Principal Investigator: Claire Dupont – LTSI
Other CAMI Partners: LTSI (Rennes), ISIR (Paris), TIMC-IMAG (Grenoble)
Started: January 2nd, 2017
Motivations and objectives:
Among mini-invasive surgical techniques, endovascular treatment consists in accessing the lesion through intravascular way to interact with pathological tissues [1]. These techniques have developed in recent years to become the reference therapy for several pathologies (aneurysm, stenosis …) [2]. However, the introduction of delivery tools through arteries and the deployment of implantable devices can have major consequences due to interactions between endovascular devices and tissues. Nowadays, the anticipation of complications mainly relies on the surgeon’s experience. Because of the regular development of more and more complex endovascular devices, the practitioners face difficulties to anticipate treatment outcome and to gain experience. To improve the planning of the treatment and optimize the interventional strategy, there is a need for new decision support tools compatible with the clinical workflow. Software tools for planning endovascular treatment already exist but mainly focus on pre-operative imaging. Beyond preoperative image analysis, explicit modelling of endovascular devices and of their interactions with tissues could be used to propose an augmented decision process by predicting possible complications [3].
Several studies about modelling and numerical simulation have been reported in the context of cardiovascular diseases. However, issues related to patient-specific simulation for endovascular treatment planning have been little addressed so far. The prediction of the mechanical equilibrium between the vascular structure and endovascular device from preoperative patient data remains a challenge.
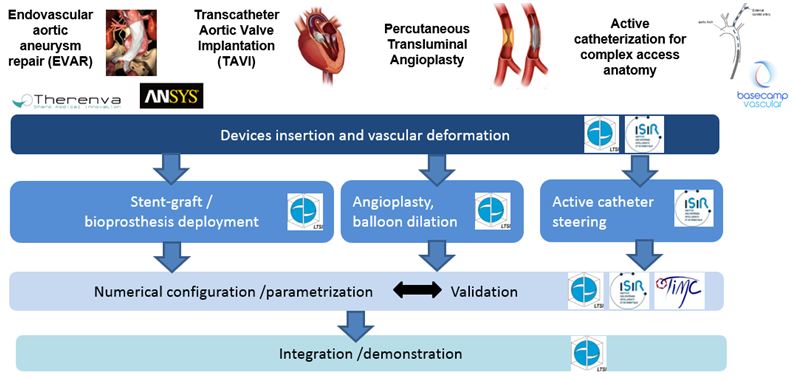
The main objectives of the project SIMPLE are to propose and develop patient-specific and predictive numerical methods to simulate endovascular treatments and to investigate their integration in the clinical decision process. Application context focuses on some endovascular interventions that share common features (e.g. delivery tools and access pathways): endovascular abdominal aneurysm repair (EVAR), transcatheter aortic valve implantation (TAVI), percutaneous transluminal angioplasty (PTA) and access to supra aortic trunks, especially in case of active catheterization.
To develop patient- specific and predictive simulation, the proposed approach is based on the joint exploitation of patient image data and biomechanical models. Work more specifically deals with the simulation of delivery tools insertion, vascular deformations, prosthesis and stent deployment, and active catheterization.
Main results and on-going work:
SIMPLE project was carried out in strong interaction with clinicians integrated in the team. First results have been obtained in the context of endovascular abdominal aneurysm repair. Work was also extended to other applications in vascular surgery, interventional cardiology and radiology.
The finite element (FE) method developed by [4] showed efficacy in predicting vascular deformation in the context of endovascular aortic aneurysm repair. However, its computational cost is a limitation to its integration into the clinical workflow. To reduce significantly the computational cost, a reduced order model (ROM) has been developed at LTSI in collaboration with ANSYS and Therenva companies. The approach consists in applying machine learning techniques [6] to reference FEM simulation results obtained from several hundred of cases.
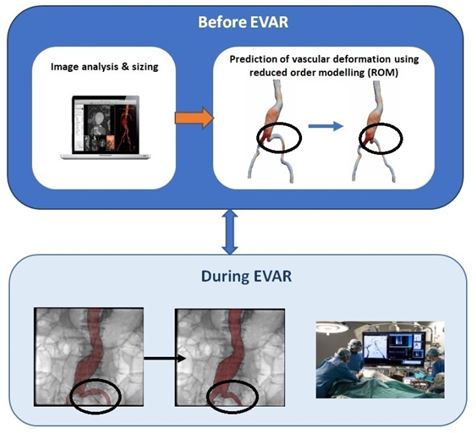
Preliminary results showed that the reduced order model could be used to estimate the vascular deformation due to the insertion of a stiff guidewire in a few seconds and could be more easily integrated into the clinical workflow. Further assessments are underway.
A numerical method has been proposed by LTSI to simulate stent-graft deployment in abdominal aortic aneurysm considering the vascular deformation due to the insertion of the delivery tools [7][8].
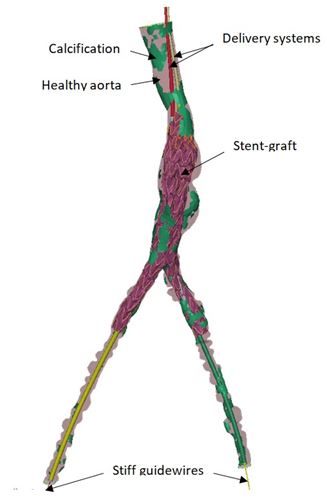
The simulation of fenestrated graft deployment could be used to anticipate stent graft (SG) and vascular structure (VS) adequacy. Main results showed that the numerically corrected fenestration positions, as determined from the simulated results following the insertion of the endovascular tools, deviate from those of the standard plan (as determined from the preoperative CT scan). This indicates that the SG–VS adequacy could be improved via simulation-based planning, to anticipate potential catheterization difficulties. Starting from preliminary results obtained in collaboration with ANSYS and other academic teams (LaMCos, Lyon; CIS, Saint Etienne) [5] [9], experiments were also conducted to adapt FE method developed for EVAR to simulate the guidewire insertion during TAVI, assuming deformable vascular structures. When it comes to patient-specific simulation of device – tissue interactions in transluminal angioplasty, the prediction of plaque behaviour remains a challenging issue. We proposed patient-specific (PS) modeling approaches for simulating percutaneous transluminal angioplasty (PTA) endovascular treatment and assessing the balloon sizing influence on short-term outcomes in peripheral arteries, i.e. without stent implantation [10][11][12]. This methodology can serve as a step toward a clinical decision support system to improve angioplasty balloon sizing selection prior to the surgery [13][14].
In order to extend simulation based decision support to active catheterization recently proposed by ISIR and Basecamp Vascular start-up, issues related to the analysis of intra-operative observations were also addressed. ISIR proposed an approach to estimate the pose and to characterize the behaviour of this new kind of devices from fluoroscopic images, more specifically when accessing supra aortic trunks [15]. TIMC developed an experimental bench, integrating fluids flow dynamics, compatible with intra-operative X-ray rotational imaging. The evaluation and the study of the influence of the blood flow on catheter navigation are then possible. This provides a tool for analysing the validity of different hypotheses eligible for catheterization simulation [16][17].
Bibliography
[1] Thompson M. M., Morgan R. A., Matsumura J. S., Sapoval M., and Loftus I. M. Endovascular Intervention for Vascular Disease: Principles and Practice. CRC Press, 2007.
[2] Wilbring M., Rehm M., Ghazy T., Amler M., Matschke K., Kappert U. Aortic Arch Mapping by Computed Tomography for Actual Anatomic Studies in Times of Emerging Endovascular Therapies. Annals of Vascular Surgery 2016;30:181–91.
[3] Kaldji A., Daoudal A., Gindre J., Lalys F., Cardon A., Haigron P., Lucas A. Numeric simulation predicts iliac complications during the implantation of an aortic stentgraft. Annals of Vascular Surgery 2017 ; 44:2.
[4] Gindre J., Bel-Brunon A., Kaladji A., Duménil A., Rochette M., Lucas A., Haigron P., Combescure A. Finite element simulation of the insertion of guidewires during an EVAR procedure: example of a complex patient case, a first step toward patient specific parameterized models: FE Simulation of the Insertion of Endovascular Guidewires. Int J Numer Method Biomed Eng. 2015 , 31, e02716.
[5] Gindre J., Bel-Brunon A., Rochette M., Lucas A., A. Kaladji, Haigron P. and Combescure A. Patient-specific finite element simulation of the insertion of guidewire during an EVAR procedure: Guidewire position and curvature prediction validation on 28 cases. IEEE Trans Biomed Eng. 2017 ; 64(5) :1057-1066.
Publications
[6] Dupont C, Boichon-Grivot C, Kaladji A, Lucas A, Rochette M, Haigron P. Statistical shape model of vascular structures with abdominal aortic aneurysm. SURGETICA’2019. Rennes, France.
[7] Dupont C., Kaladji A., Gindre J., Lucas A., Haigron P. Patient specific finite element simulation of stent-graft deployment in EVAR procedure. SURGETICA’2017. Strasbourg, France. Nov. 20-22 2017.
[8] Dupont C, Kaladji A, Rochette M, Saudreau B, Lucas A, Haigron P. Numerical Simulation of Fenestrated Graft Deployment: Anticipation of Stent Graft and Vascular Structure Adequacy. International Journal for Numerical Methods in Biomedical Engineering. 2021 Jan;37(1): e03409.
[9] P. Vy, V. Auffret, M. Castro, P. Badel, M. Rochette, P. Haigron, S. Avril. Patient‐specific simulation of guidewire deformation during transcatheter aortic valve implantation. Int J Numer Method Biomed Eng. 2018.
[10] Al-Helou B, Dupont C, Bel-Brunon A, Ye W, Kaladji A, Haigron P. Towards a patient‐specific simulation of the balloon angioplasty treatment technique. SURGETICA’2019. Rennes, France.
[11] Al Helou B, Bel-Brunon A, Dupont C, Ye WF, Silvestro C, Rochette M, Lucas A, Kaladji A, Haigron P. Influence of balloon design, plaque material composition, and balloon sizing on acute post angioplasty outcomes: An implicit finite element analysis. Int. Journal for Numerical Methods in Biomedical Engineering. 2021 August;37(8): e03499.
[12] Al Helou B. Modeling and numerical simulation for the planning of percutaneous transluminal angioplasty. Thèse de doctorat de l’Université de Rennes. Juillet 2021.
[13] Joly C, Bel-Brunon A, Kaladji A, Haigron P. A parametric study assessing Implicit Solver limits for a generic FEM Simulation of PTA without stent deployment. 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). 2023 Jul;2023:1-4.
[14] Al Helou B, Bel-Brunon A, Dupont C, Ye WF, Silvestro C, Rochette M, Lucas A, Kaladji A, Haigron P. Patient-specific finite element simulation of peripheral artery percutaneous transluminal angioplasty to evaluate the procedure outcome without stent implantation.
Int. Journal for Numerical Methods in Biomedical Engineering. 2023 March; 39(3): e3685.
[15] En‐Tzu Yang A, Szewczyk J. Investigating the Role of Helical Markers in 3D Catheter Shape Monitoring from 2D Fluoroscopy. SURGETICA’2019. Rennes, France.
[16] Lagache M, Coppel R, Gomez A, Finet G, Ohayon J. Experimental test bench for the hemodynamic study of coronary arteries: bifurcation, stent, aneurysm. SURGETICA’2019. Rennes, France.
[17] Lagache M, Coppel R, Finet G, Derimay F, Pettigrew R, Ohayon J, Malve M. Impact of Malapposed and Overlapping Stents on Hemodynamics: A 2D Parametric Computational Fluid Dynamics Study. Mathematics. 2021 ; 9: 795.
SIMPLE team:
- Bernard Al Helou (LTSI)
- Claire Dupont (LTSI)
- Pascal Haigron (LTSI)
- Adrien Kaladji (LTSI)
- Manuel Lagache (TIMC)
- Antoine Lucas (LTSI)
- Jérôme Szewczyk (ISIR)


1 thought on “SIMPLE”
Comments are closed.